Recientemente se ha publicado en la ‘Jounal of Renewable and Sustainable Energy’ (AIP) una investigación de científicos de la Academia de Ciencias de China y la Universidad de Tsinghua. En esta publicación, se hace referencia a que este grupo ha probado con éxito un método para generar hidrógeno in situ y a demanda, a partir de una aleación de metales.
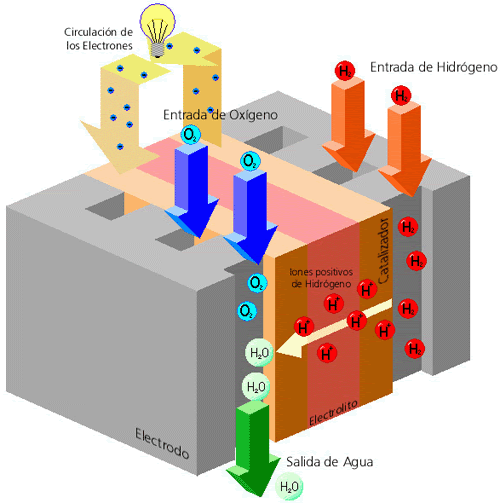
El funcionamiento de una pila de hidrógeno se fundamenta en una membrana polimérica que separa el lado del ánodo del lado del cátodo. En el ánodo el hidrógeno se disocia en protones y electrones con la ayuda de un catalizador. Los protones son conducidos a través de la membrana hacia el cátodo mientras los electrones son forzados a viajar a través de un circuito externo donde se produce la energía. En el catalizador del cátodo las moléculas de oxígeno reaccionan con los electrones y protones formando agua de modo que el único residuo generado es vapor de agua o agua liquida.
Los científicos han descubierto que para producir Hidrógeno, mediante la reacción del Aluminio con el Agua, si se activa a partir de una nueva aleación de Galio, Indio, Estaño y Bismuto se puede obtener con una productividad del 92%.
Los científicos han descubierto un mecanismo para activar la producción de hidrógeno en tiempo real, bajo demanda de los usuarios. Por el momento se trata de un proyecto a pequeña escala, pero abre la puerta a producir en un futuro hidrógeno de manera estable, con una alta pureza, una alta eficiencia y sin problemas de degradación que al final complican y encarecen el transporte.


